Rationale: Messenger RNA (mRNA) vaccine outperforms other kinds of cancer immunotherapy due to its
high response rates, easy preparation, and wide applicability, which is considered as one of the most promising
forms of next-generation cancer therapies. However, the inherent instability and insufficient protein
expression duration of mRNA limit the efficacy and widespread application of the vaccine.
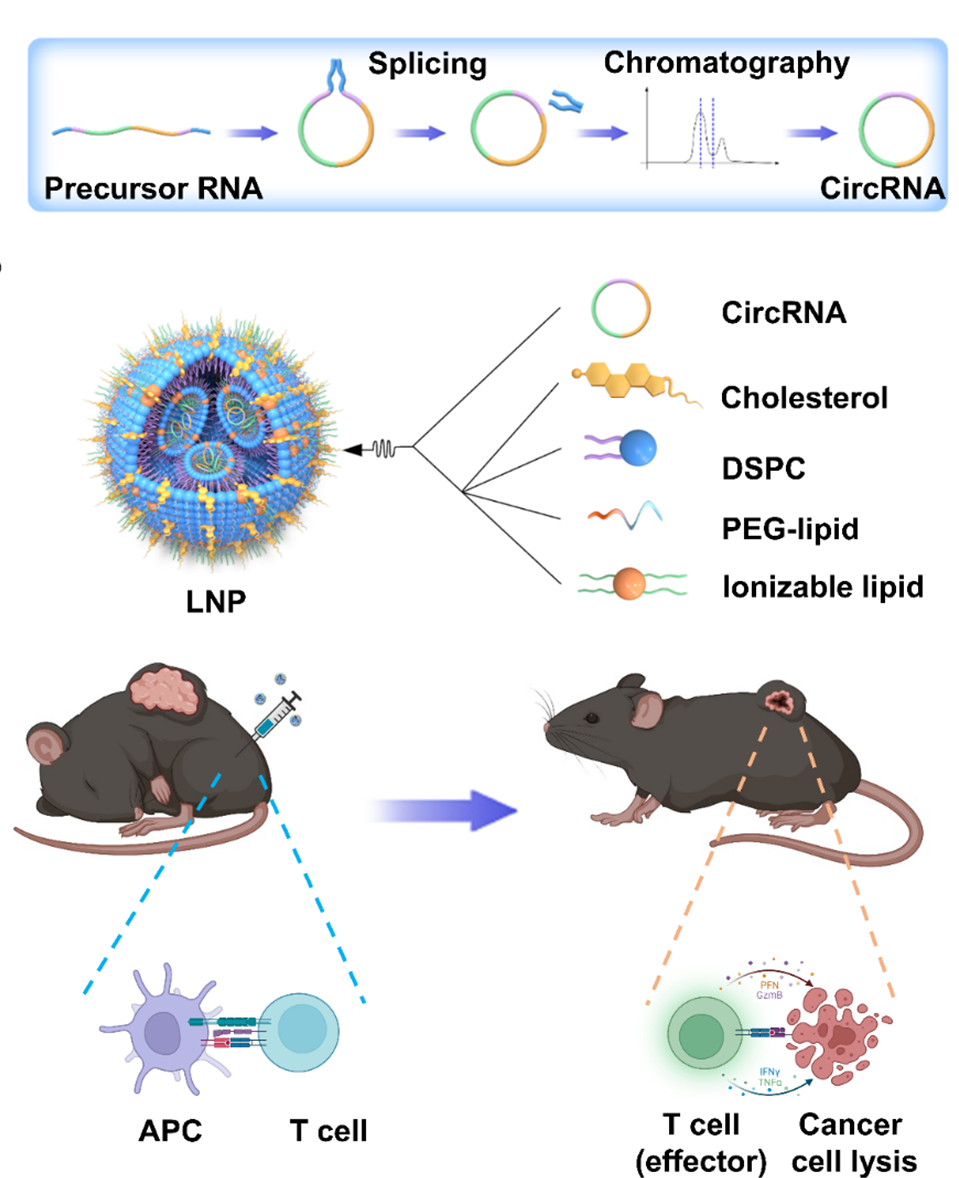
Methods: Here, we first tested the possibility of a novel circular RNA (circRNA) platform for protein
expression and compare its duration with linear RNA. Then, we developed a lipid nanoparticle (LNP) system
for circRNA delivery in vitro and in vivo. Next, the innate and adaptive immune response of circRNA-LNP
complex was evaluated in vivo. The anti-tumor efficacy of circRNA-LNP was further confirmed in three tumor
models. Finally, the possibility of combination therapy with circRNA-LNP and adoptive cell transfer therapy
was further investigated in a late-stage tumor model.
Results: We successfully increased the stability of the RNA vaccine by circularizing the linear RNA molecules
to form highly stable circRNA molecules which exhibited durable protein expression ability. By encapsulating
the antigen-coding circRNA in LNP enabling in vivo expression, we established a novel circRNA vaccine
platform, which was capable of triggering robust innate and adaptive immune activation and showed superior
anti-tumor efficacy in multiple mouse tumor models.
Conclusions: Overall, our circRNA vaccine platform provides a novel prospect for the development of
cancer RNA vaccines in a wide range of hard-to-treat malignancies.