Fangfang Cao 1 2, Lulu Jin 3, Chenyin Zhang 3, Yong Gao 3, Zhefeng Qian 3 4, Hongyang Wen 3 4, Sisi Yang 5, Ziqiang Ye 3, Liangjie Hong 3, Huang Yang 3, Zongrui Tong 4, Liang Cheng 6, Yuan Ding 4, Weilin Wang 4, Guocan Yu 7, Zhengwei Mao 3 4, Xiaoyuan Chen 1 2 8 9
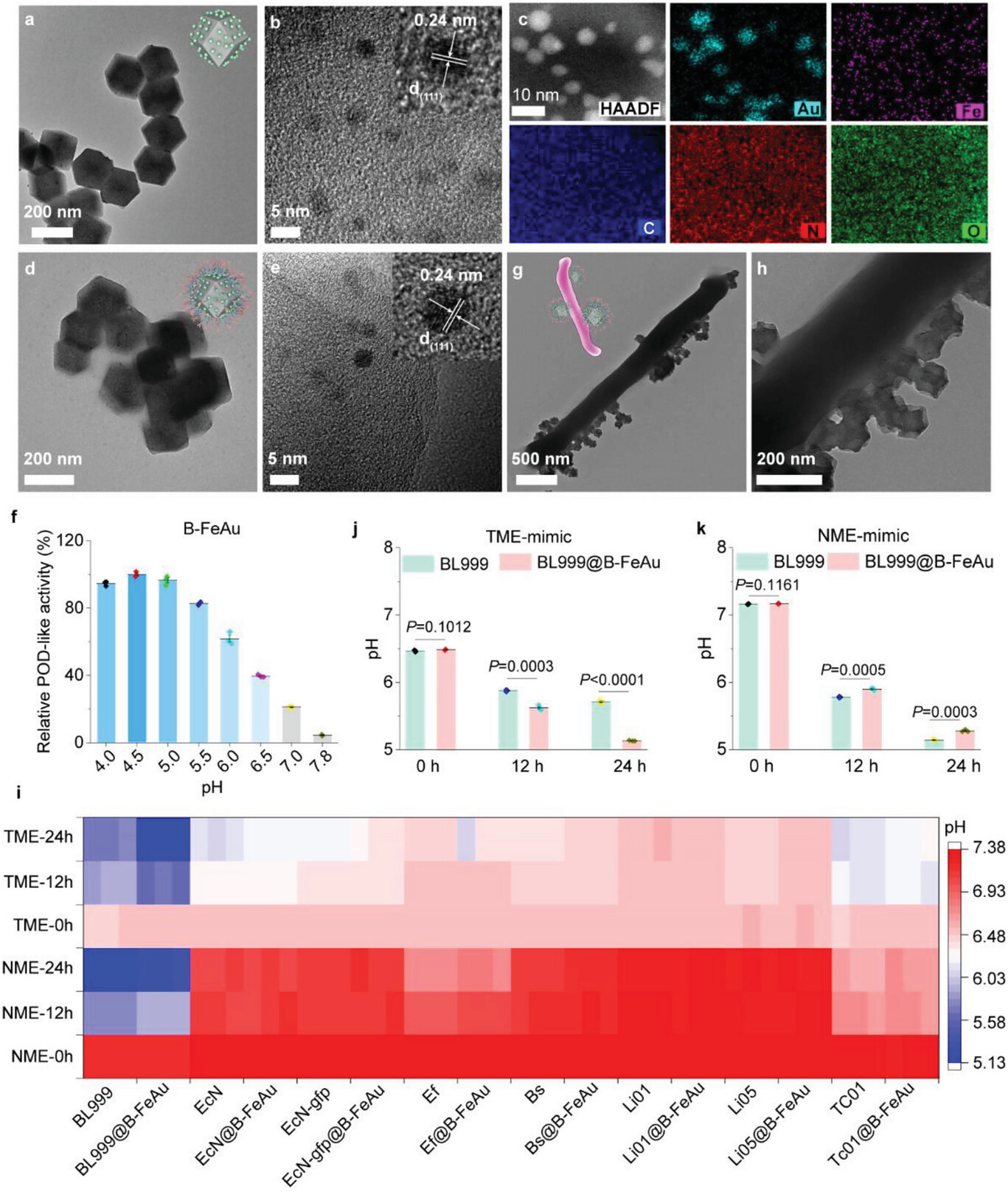
Probiotics have the potential as biotherapeutic agents for cancer management in preclinical models and human trials by secreting antineoplastic or immunoregulatory agents in the tumor microenvironment (TME). However, current probiotics lack the ability to dynamically respond to unique TME characteristics, leading to limited therapeutic accuracy and efficacy. Although progress has been made in customizing controllable probiotics through synthetic biology, the engineering process is complex and the predictability of production is relatively low. To address this, here, for the first time, this work adopts pH-dependent peroxidase-like (POD-like) artificial enzymes as both an inducible “nano-promoter” and “nano-effector” to engineer clinically relevant probiotics to achieve switchable control of probiotic therapy. The nanozyme initially serves as an inducible “nano-promoter,” generating trace amounts of nonlethal reactive oxygen species (ROS) stress to upregulate acidic metabolites in probiotics. Once metabolites acidify the TME to a threshold, the nanozyme switches to a “nano-effector,” producing a great deal of lethal ROS to fight cancer. This approach shows promise in subcutaneous, orthotopic, and colitis-associated colorectal cancer tumors, offering a new methodology for modulating probiotic metabolism in a pathological environment.